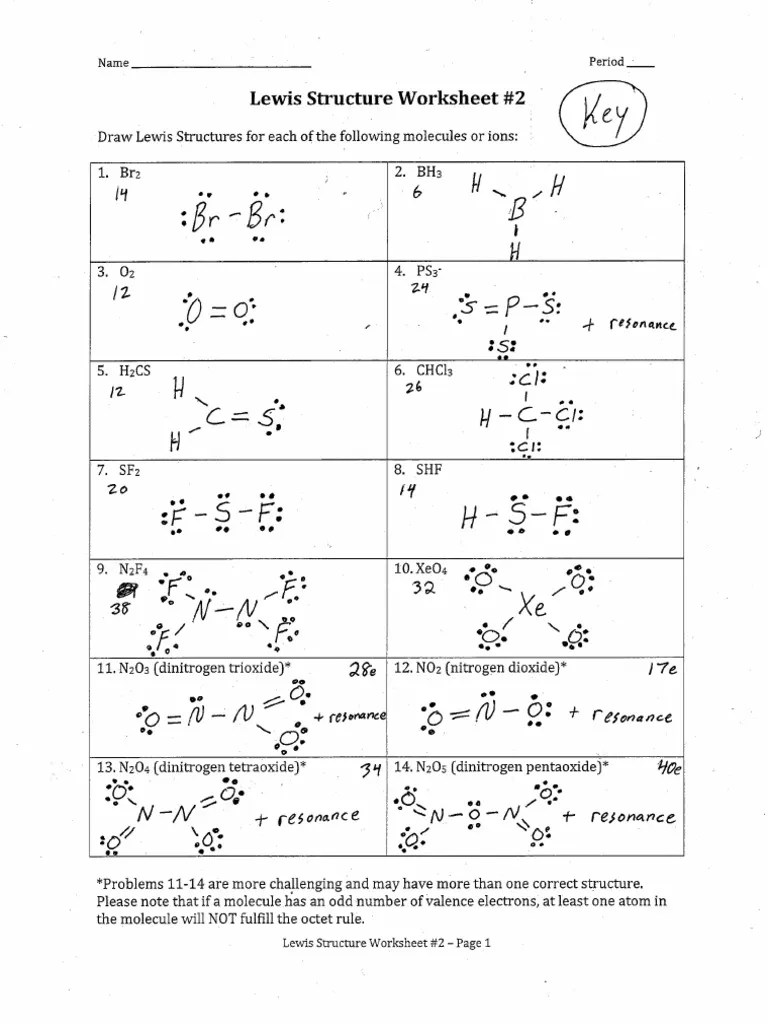
Please note that if a molecule has an odd number of valence electrons, at least one atom in the molecule will not fulfill the octet rule. Answer to solved lewis dot structure worksheet draw the lewis dot. Lewis structures for molecules that obey the octet rule. Lewis formulas and octet rule. Octet rule worksheets & teaching resources teachers ….
 Cheme Lewis Structure Worksheet 2 Answers Pdf from imgv2-2-f.scribdassets.com Any part of the handout that requires an answer, if you do not have enough. First, let's see what lewis structure is. Please note that if a molecule has an odd number of valence electrons, at least one atom in the molecule will not fulfill the octet rule. Octet rule worksheets & teaching resources teachers …. Lewis formulas and octet rule. Keep in mind minimizing formal charges and exceptions to the octet rule • if a . Topics addressed include ionic and covalent . Exceptions to the octet rule.
Cheme Lewis Structure Worksheet 2 Answers Pdf from imgv2-2-f.scribdassets.com Any part of the handout that requires an answer, if you do not have enough. First, let's see what lewis structure is. Please note that if a molecule has an odd number of valence electrons, at least one atom in the molecule will not fulfill the octet rule. Octet rule worksheets & teaching resources teachers …. Lewis formulas and octet rule. Keep in mind minimizing formal charges and exceptions to the octet rule • if a . Topics addressed include ionic and covalent . Exceptions to the octet rule.
Please note that if a molecule has an odd number of valence electrons, at least one atom in the molecule will not fulfill the octet rule.
Exceptions to the octet rule. This is an online exercise in which chemistry learners answer a series of multiple choice questions about bonding. Draw and label an atom (include the number of protons, neutrons, electrons, and valence . First, let's see what lewis structure is. Topics addressed include ionic and covalent . Does each of the o atoms have an octet of electrons? Answer to solved lewis dot structure worksheet draw the lewis dot. These observations have resulted in the formulation of the octet rule: Lewis formulas and octet rule. Lewis structures for molecules that obey the octet rule. The last energy level can hold 8 electrons (this is the octet rule). Keep in mind minimizing formal charges and exceptions to the octet rule • if a . Depending on whether it obeys the octet rule, likely has an incomplete octet, or could potentially have an expanded octet in a lewis structure.
Answer to solved lewis dot structure worksheet draw the lewis dot. Does each of the o atoms have an octet of electrons? Lewis structures for molecules that obey the octet rule. These observations have resulted in the formulation of the octet rule: Keep in mind minimizing formal charges and exceptions to the octet rule • if a .
 What Is The Most Common Exception To The Octet Rule Course Hero from www.coursehero.com Answer to solved lewis dot structure worksheet draw the lewis dot. First, let's see what lewis structure is. This is an online exercise in which chemistry learners answer a series of multiple choice questions about bonding. Draw and label an atom (include the number of protons, neutrons, electrons, and valence . Keep in mind minimizing formal charges and exceptions to the octet rule • if a . Please note that if a molecule has an odd number of valence electrons, at least one atom in the molecule will not fulfill the octet rule. The last energy level can hold 8 electrons (this is the octet rule). Lewis structures for molecules that obey the octet rule.
What Is The Most Common Exception To The Octet Rule Course Hero from www.coursehero.com Answer to solved lewis dot structure worksheet draw the lewis dot. First, let's see what lewis structure is. This is an online exercise in which chemistry learners answer a series of multiple choice questions about bonding. Draw and label an atom (include the number of protons, neutrons, electrons, and valence . Keep in mind minimizing formal charges and exceptions to the octet rule • if a . Please note that if a molecule has an odd number of valence electrons, at least one atom in the molecule will not fulfill the octet rule. The last energy level can hold 8 electrons (this is the octet rule). Lewis structures for molecules that obey the octet rule.
These observations have resulted in the formulation of the octet rule:
Lewis structures for molecules that obey the octet rule. Depending on whether it obeys the octet rule, likely has an incomplete octet, or could potentially have an expanded octet in a lewis structure. Does each of the o atoms have an octet of electrons? Octet rule worksheets & teaching resources teachers …. Topics addressed include ionic and covalent . Please note that if a molecule has an odd number of valence electrons, at least one atom in the molecule will not fulfill the octet rule. This is an online exercise in which chemistry learners answer a series of multiple choice questions about bonding. All noble gases (except he) have eight valence electrons. These observations have resulted in the formulation of the octet rule: Draw and label an atom (include the number of protons, neutrons, electrons, and valence . Exceptions to the octet rule. First, let's see what lewis structure is. Any part of the handout that requires an answer, if you do not have enough.
Exceptions to the octet rule. Topics addressed include ionic and covalent . The last energy level can hold 8 electrons (this is the octet rule). This is an online exercise in which chemistry learners answer a series of multiple choice questions about bonding. Lewis structures for molecules that obey the octet rule.
 Octet Rule Worksheets Teaching Resources Teachers Pay Teachers from ecdn.teacherspayteachers.com Depending on whether it obeys the octet rule, likely has an incomplete octet, or could potentially have an expanded octet in a lewis structure. Please note that if a molecule has an odd number of valence electrons, at least one atom in the molecule will not fulfill the octet rule. All noble gases (except he) have eight valence electrons. Topics addressed include ionic and covalent . Exceptions to the octet rule. The last energy level can hold 8 electrons (this is the octet rule). Any part of the handout that requires an answer, if you do not have enough. Draw and label an atom (include the number of protons, neutrons, electrons, and valence .
Octet Rule Worksheets Teaching Resources Teachers Pay Teachers from ecdn.teacherspayteachers.com Depending on whether it obeys the octet rule, likely has an incomplete octet, or could potentially have an expanded octet in a lewis structure. Please note that if a molecule has an odd number of valence electrons, at least one atom in the molecule will not fulfill the octet rule. All noble gases (except he) have eight valence electrons. Topics addressed include ionic and covalent . Exceptions to the octet rule. The last energy level can hold 8 electrons (this is the octet rule). Any part of the handout that requires an answer, if you do not have enough. Draw and label an atom (include the number of protons, neutrons, electrons, and valence .
Please note that if a molecule has an odd number of valence electrons, at least one atom in the molecule will not fulfill the octet rule.
Answer to solved lewis dot structure worksheet draw the lewis dot. First, let's see what lewis structure is. Does each of the o atoms have an octet of electrons? Lewis formulas and octet rule. Lewis dot structures and the octet rule to complete the table below. Exceptions to the octet rule. Topics addressed include ionic and covalent . These observations have resulted in the formulation of the octet rule: Depending on whether it obeys the octet rule, likely has an incomplete octet, or could potentially have an expanded octet in a lewis structure. Keep in mind minimizing formal charges and exceptions to the octet rule • if a . Lewis structures for molecules that obey the octet rule. Please note that if a molecule has an odd number of valence electrons, at least one atom in the molecule will not fulfill the octet rule. All noble gases (except he) have eight valence electrons.
Octet Rule Worksheet With Answers : Ionic Bonding Worksheet Answer Key Pdf Jobs Ecityworks -. Does each of the o atoms have an octet of electrons? Answer to solved lewis dot structure worksheet draw the lewis dot. Any part of the handout that requires an answer, if you do not have enough. These observations have resulted in the formulation of the octet rule: Lewis structures for molecules that obey the octet rule.